Hit ENTER to search or ESC to close

This section is dedicated to medical professional. Here you can review NeuroAiD’s clinical data, read about its pharmacology, go through publications, and assess the relevance of NeuroAiD in your practice and for your stroke and traumatic brain injured patients.
For more information, do not hesitate to contact us by email at medical@moleac.com
*Note: NeuroAiD™ (MLC601) is the original formula composed by 14 ingredients. NeuroAiD™ II and NurAiD™ II (MLC901) are simplified formulas of NeuroAiD™ (MLC601) composed with the 9 herbal ingredients of the original formula. MLC901 and MLC601 have been shown to be pharmacologically equivalent. In the interest of simplification, the following information will use NeuroAiD as a global name to designate the products except in case of specific mentions.
Following national regulations in each country, NeuroAiD is registered under various schemes, which includes dietary supplement, traditional medicine and herbal medicine. To date, NeuroAiD is available in over 32 countries worldwide and is continuously expanding its development.
With the support and involvement of international key opinion leaders in Neurology and experienced researchers, NeuroAiD has gathered over 45 publications from pre-clinical studies to randomized clinical trials, and aims at addressing a worldwide medical need.

The recommended dosage is 2 capsules at a time, 3 times daily, for a minimum of 3 months.
Oral route with a glass of water. For people with swallowing difficulties, it is possible to open the capsules and to dilute the content in water. It is also possible to administer the diluted content via a gastric tube.
To date, no drug interactions with NeuroAiD have been reported. For more information on NeuroAiD’s safety profile, click HERE.
Published clinical trials have confirmed that NeuroAiD has no blood thinning effect or effect on liver or renal functions. Similarly, NeuroAiD has neither any effect on blood pressure, and doesn’t induce any change in cardiac, hematological, hemostatic and biochemical parameters.1,2
Clinical trials on NeuroAiD reported only rare cases of nausea, minor headaches, increased thirst, dry mouth and vomiting for patients taking NeuroAiD. In these cases, it is advised to halve the dose of NeuroAiD for a few days and progressively increase it.
The use of NeuroAiD in pregnant women, lactating women and children is not documented, and cannot be recommended. To date, no other contraindications are known.
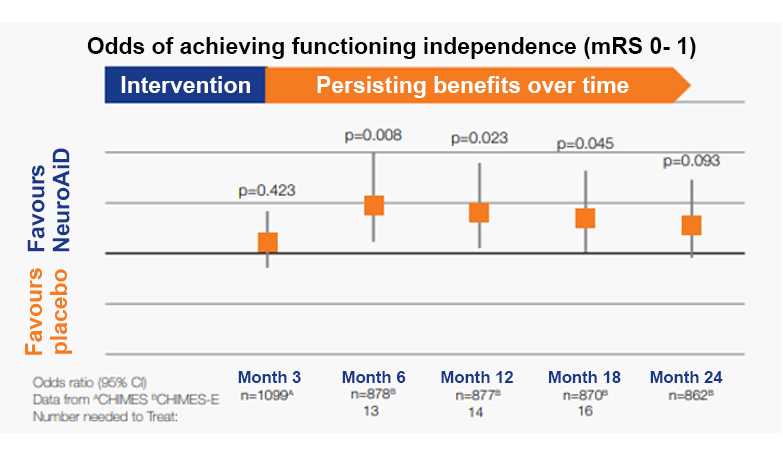
+50% in odds of achieving functional independence at 6 months.
Persistent recovery with benefits observed over 2 years
*CHIMES-E published in Cerebrovascular Diseases journal is the pre-planned extended follow-up to 24 months of the CHIMES study5, a 3-month study conducted on 1099 subjects. The CHIMES study was published in Stroke journal in 2013. **Prognostic factors are defined as older age, female sex, baseline NIHSS score ≥ 10 and time to first dose 48h.
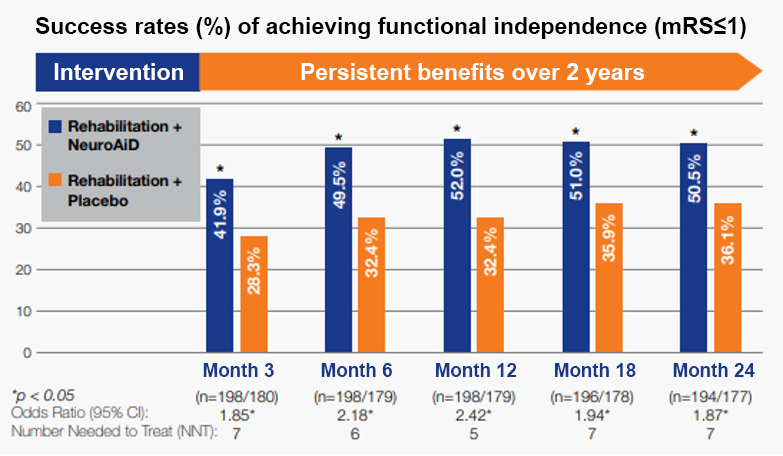
+50% in odds of achieving functional independence at 6 months.
Persistent recovery with benefits observed over 2 years
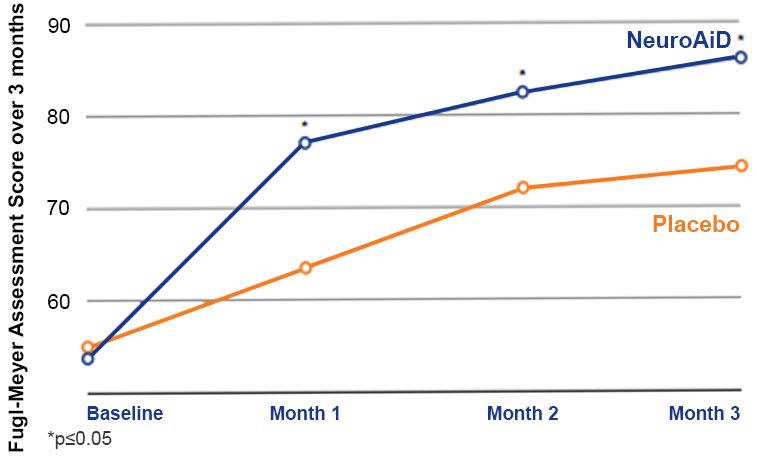
Significantly better motor recovery observed since month 1 and sustained up to month 3.
References:
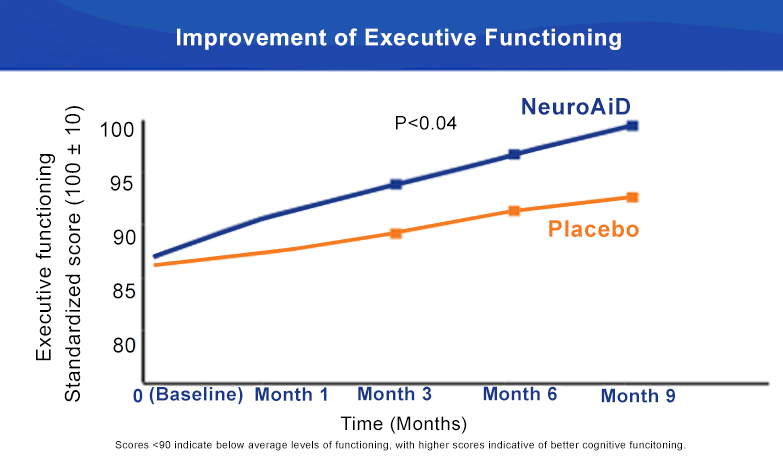
NeuroAiD significantly improves Executive Functioning and Complex Attention at 6 months.
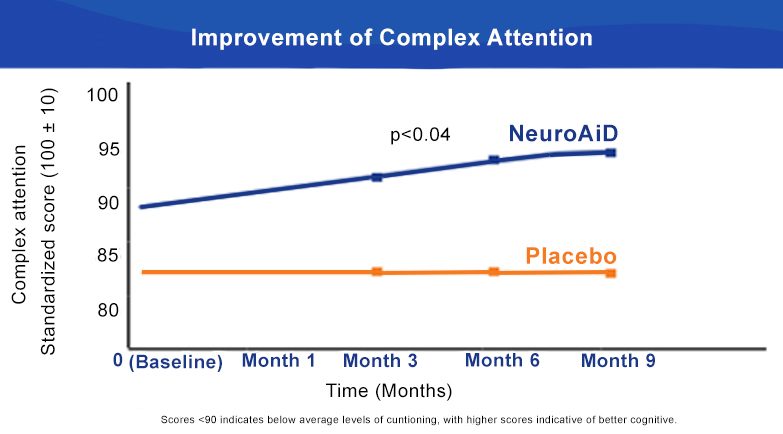
NeuroAiD optimises recovery of moderate and severe TBI subjects over time
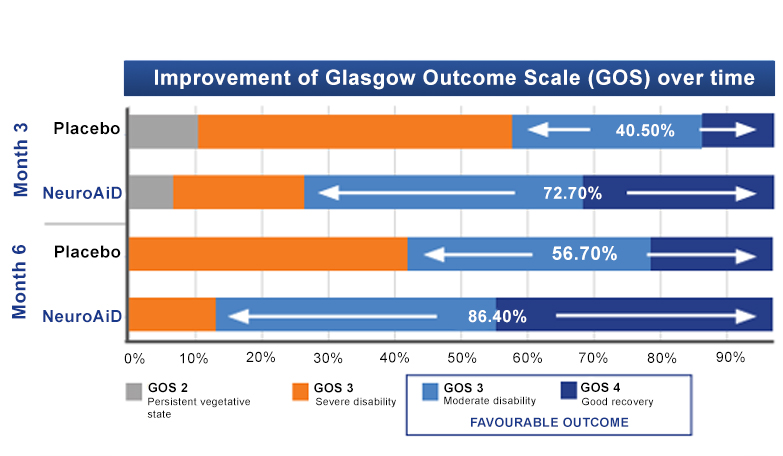
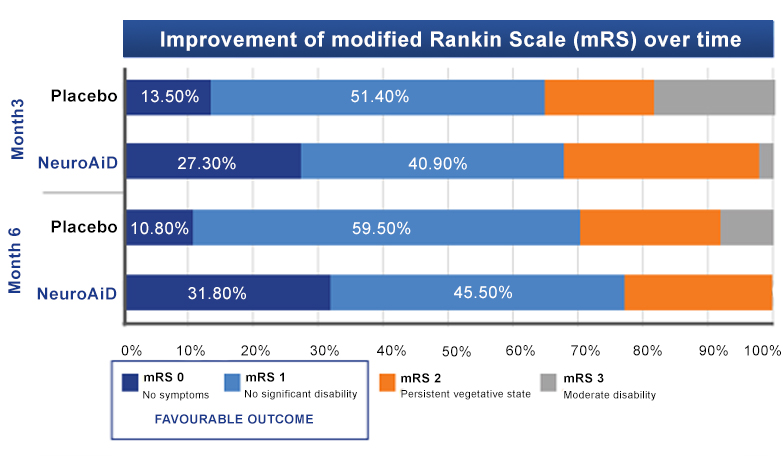
Visit the Publicationstab
for more information on NeuroAiD’s clinical data
To date, no drug interactions with NeuroAiD have been reported. NeuroAiD should be taken in addition to preventive treatments of cardiovascular risk factors commonly prescribed after a stroke; these typically include antiplatelet, antihypertensive, and cholesterol-lowering drugs, among others such as anti-diabetics. Systematic clinical trials have shown that NeuroAiD does not have any interaction with aspirin. Patients taking NeuroAiD with anticoagulants have reported no impact on their INR (International Normalized Ratio). Nevertheless, it is recommended to keep monitoring INR when NeuroAiD is initiated as it is routinely done for patients taking anticoagulants.
NeuroAiD is manufactured in Singapore under the international standards of Good Manufacturing Practice (GMP). The GMP standards are applied to procedures in manufacturing dietary supplements, including approval of raw materials, production and process controls, laboratory testing, and product packaging, labelling, and distribution. These processes are regularly audited by independent health authorities. Every batch of NeuroAiD is analysed thoroughly by third-party independent laboratories to confirm compliance with international standards, ensuring utmost ensuring utmost security of NeuroAiD use.
References:
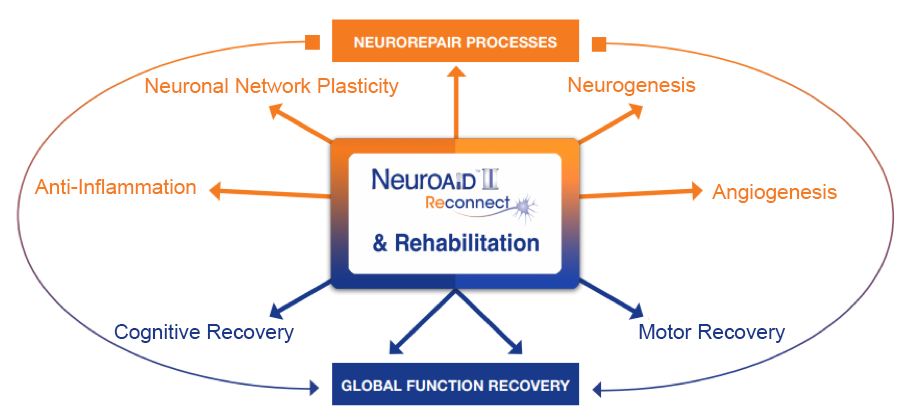
2. NEUROGENESIS¹²
3. ANGIOGENSIS³
4. NEURO-INFLAMMATION MODULATION⁴
BDNF is a growth factor which regulates neuronal survival and protects neurons from glutamate-induced damages. BDNF also encourages proliferation and differentiation of new neurons.
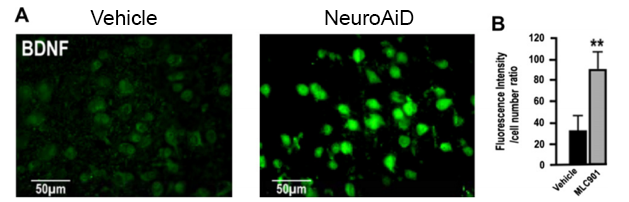
In vivo effect of NeuroAiD pre-treatment on BDNF protein levels in cortex sections. (A) Representative epifluorescence microscopy photographs of BDNF immunoexpression in cortical neurons in brain sections. (B) Quantification of BDNF signal intensity in immunostained neurons.
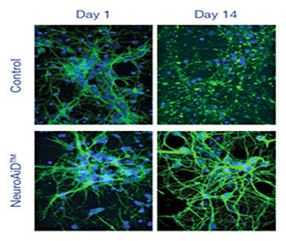
Epifluorescence microscopy confirmed the development of an important axonal and dendritic network on cell cultures with NeuroAiD.
On day 14, in neuronal cell cultures treated with NeuroAiD, epifluorescence showed a remarkable increase of DCX, GAP43 and Synaptotagmin expressions, providing evidence that NeuroAiD triggers:
NeuroAiD promotes both the development of new synaptic connections and the modification of existing ones, including an increase in the number and length of dendrites and synapses. A 60% increase in the length of the neurite was shown at Day 14 for NeuroAiD group.
Effects of MLC601/MLC901 treatment on in vitro Synaptotagmin 1 expression in cultured cortical neurons. Synaptotagmin 1 expression was analyzed after MLC601/MLC901 treatment (1 mg/ml) at Day 7 and 14 of treatment.
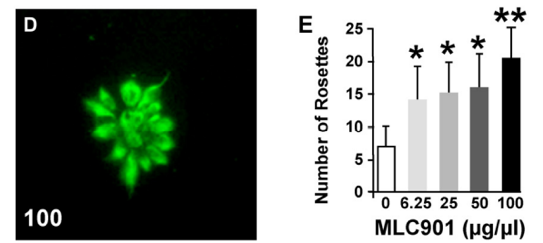
Neurogenic effects of MLC901 on human ESC-derived progenitors. (D) Radiant rosette-like aggregates of nestin-positive neural progenitors spontaneously form in low-density cultures. (E) Quantification of the number of rosettes 2 days after the addition of MLC901 (*P < 0.05, **P < 0.01 versus control).
Visit the Publications tab for more information on NeuroAiD’s properties and mode of action.
References:
Alzheimer’s Disease (AD) results from a neurodegenerative process linked to various causes and involving several pharmacological pathways. With its multimodal mechanism of action, NeuroAiD provides an innovative approach to Alzheimer’s treatment.
NeuroAiD has a multimodal mode of action on the hallmarks of Alzheimer’s Disease mechanisms.
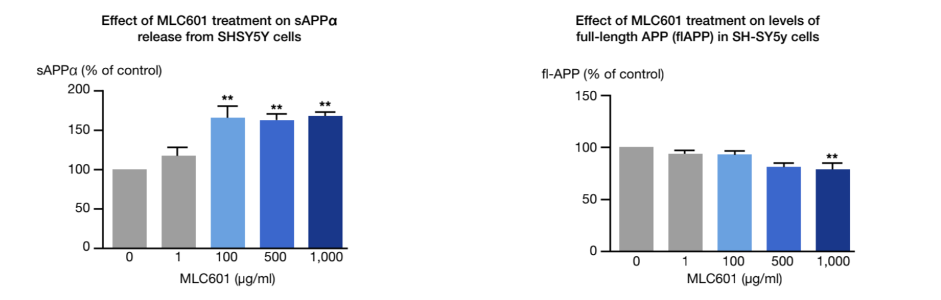
Under pathological conditions such as AD, the amyloidogenic pathway of amyloid precursor protein (APP) processing results in large amount of the non-soluble and neurotoxic Aβ fragments, inducing the formation of neurofibrillary tangles. A therapeutic approach would target the APP processing to limit the amyloidogenic pathway.
An in-vitro study conducted on cultures of human neuroblastoma cells showed the effect of NeuroAiD on the APP processing 1.
The hyperphosphorylation of the tau proteins is another neuropathological hallmark of AD. An in-vivo study showed that NeuroAiD is impacting this process 2.
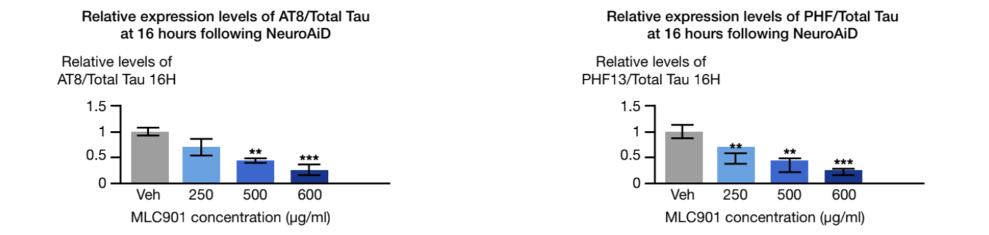
AT8: monoclonal antibody used in western blotting to measure tau phosphorylation
PHF: paired helical filament used in western blotting to measure tau phosphorylation
The ATHENE study 3
A randomised, double-blind, placebo-controlled trial conducted on 125 mild-to-moderate probable AD subjects, receiving NeuroAiD (MLC901, 2 capsules, 3 times a day) or placebo as an add-on therapy to standard AD treatment for 6 months, followed by an open-labelled extension study where all subjects received NeuroAiD in addition to standard of care.
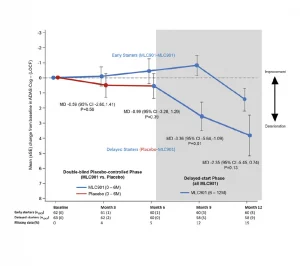
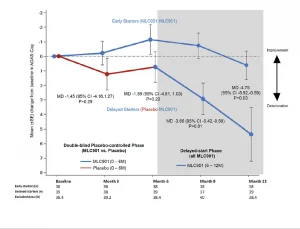
Open-label pilot study including 124 Alzheimer’s disease patients who failed to benefit from a previous 6-month course of Rivastigmine (lack of efficacy and/or poor tolerability), receiving NeuroAiD for 4 years. Cognitive state of the subjects was assessed by MMSE and ADAS-Cog every 6 months.4*,5*
In a multicentre, randomized, controlled trial conducted on 264 patients with mild to moderate Alzheimer’s Disease, NeuroAiD was compared to 3 approved cholinesterase inhibitors (i.e. Donepezil, Rivastigmine, Galantamine) for 16 months.6*
References
* Các nghiên cứu được tiến hành với NeuroAiD MLC601 với liều 1 viên, 3 lần một ngày.
NeuroAiDTM là thương hiệu của Moleac. MLC601 (NeuroAiDTM) và MLC901 (NeuroAiDTTMI/ NurAiDTTMI) là hai công thức độc quyền khác nhau đã được chứng minh là tương đương về mặt dược lý học và được gọi là “NeuroAiD” trong tài liệu này.
Moleac has developed multiple partnerships with companies sharing the same values and vision to deliver innovative solutions in each market and address medical unmet needs, particularly in post-stroke and post-traumatic brain injury recovery.
Our growing network of partners ensures the availability of NeuroAiD to an even greater number of stroke victims and victims of traumatic brain injuries worldwide but also patients experiencing cognitive decline.
Since 2006, NeuroAiD has expended its presence to over 32 countries and we still aim to make NeuroAiD available to more patients in need worldwide.
 Offices
Offices Partners
Partners